Valvular heart disease is a serious condition that afflicts millions of patients worldwide, and approximately 250,000 heart valve related procedures are performed each year. Heart valves affected by valvular heart disease suffer from reduced hemodynamic performance in two primary ways, forward flow and reverse flow. These are classified as stenosis and incompetence respectively. While both conditions require surgical implantation of a heart valve prosthesis to restore normal cardiac function, there are many different valves available to choose from based on which native valve is being replaced and viability for the patient.
Here at the Heart Valve Performance Laboratory, we are researching and developing a wide range of prosthetic heart valves for all positions including mechanical, pediatric, percutaneous, and bioprosthetic. These projects are all being developed concurrently, with lessons learned and discoveries being shared to nurture a rapid development process. In addition to our work on heart valves, we are developing a novel new way to test these prosthetics in-house to shed light on the mechanics of heart valves in vitro. Below you can find further information on our heart valve projects and individually dive deeper into the research being done right here in the heart of the Okanagan.
Mechanical heart valves are the oldest and most robust prosthetic option available for patients afflicted by valvular heart disease. Their implantation requires open-heart surgery to be performed on a stopped heart and for a patient to be placed on a heart-lung bypass machine. While this isn’t an option for all patients, it allows for precise and secure implantation of the prosthesis. Mechanical heart valves have been used to replace diseased valves since their advent in 1952 but some limitations of the earliest designs still persist today despite decades of research and innovation.
Here at the Heart Valve Performance Laboratory, we are working towards designing the next generation of mechanical heart valves with the goal of creating a valve with ideal hemodynamics that can be implanted without any reliance on anticoagulants. In other words, a perfect replacement for native heart valves.
Pediatric heart valves, as the name suggests, are heart valves specifically designed to be implanted within the hearts of children. While many adult patients have their valves replaced due to the degenerative processes of valvular heart disease, pediatric valves are implanted to correct valves that didn’t develop properly due to congenital heart defects. In 2018, Abbott Laboratories received FDA approval for their pediatric heart valve and it still remains as the only heart valve available in a pediatric size. Before 2018, children requiring a heart valve replacement had to also undergo dangerous enlargement operations to fit the smallest available adult-sized heart valves.
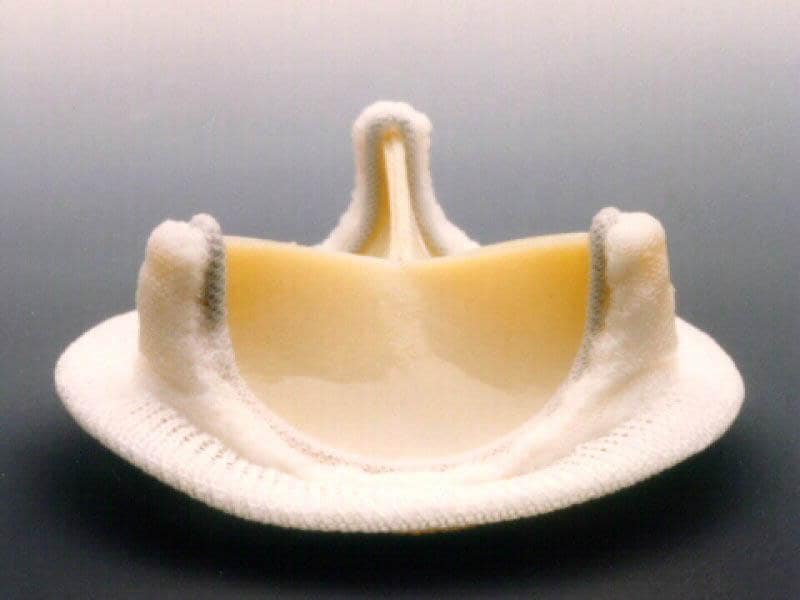
Pediatric heart valves being constrained to one valve design made by one manufacturer, however, is not an ideal situation. The unmet need for a valve designed specifically for the hemodynamic conditions of a child’s heart still remains, and this is where the Heart Valve Performance Laboratory’s research is focused, developing a valve to operate under the accelerated conditions of a child’s heart. Depicted to the left is a soft robotic heart valve being developed by our team that has demonstrated unique hemodynamics performance characteristics at miniature annulus sizes.
Percutaneous heart valves (also known as transcatheter heart valves) are a catheter-based heart valve that is implanted while the patient’s heart is still beating. For patients who are not suited to survive open-heart surgery, this option is essential to survival. The first development of percutaneous heart valves began in the late 1980s and has quickly become a major component of cardiovascular engineering.
Developments of commercialized percutaneous aortic and pulmonary valves have led to the development of percutaneous mitral valves. This technology is relatively new, and cutting edge developments are being made constantly. The future goal is to be able to eliminate open-heart surgery, by producing percutaneous valves that can rival the performance of surgical valves.
Bioprosthetic heart valves are valves made from animal pericardial tissue that are surgically implanted within patients. Surgical aortic valve replacement (SAVR) is the procedure performed to replace compromised aortic valves with bioprosthetic heart valves and has a high surgical risk as it is open-heart surgery. This requires cutting open the patient’s chest to expose the heart and placing the patient on a heart-lung bypass machine while the heart is stopped and the procedure is performed.
Bioprosthetic heart valves come in two different forms, stented and stentless. Stented valves have leaflets fixed within a rigid stent which can then be implanted into most patients. Stentless valves are different such that the aortic root is replaced by a donor root with a valve within it. The major benefit of stentless valves is their large effective orifice area and related low incidence rate of patient-prosthesis mismatch.
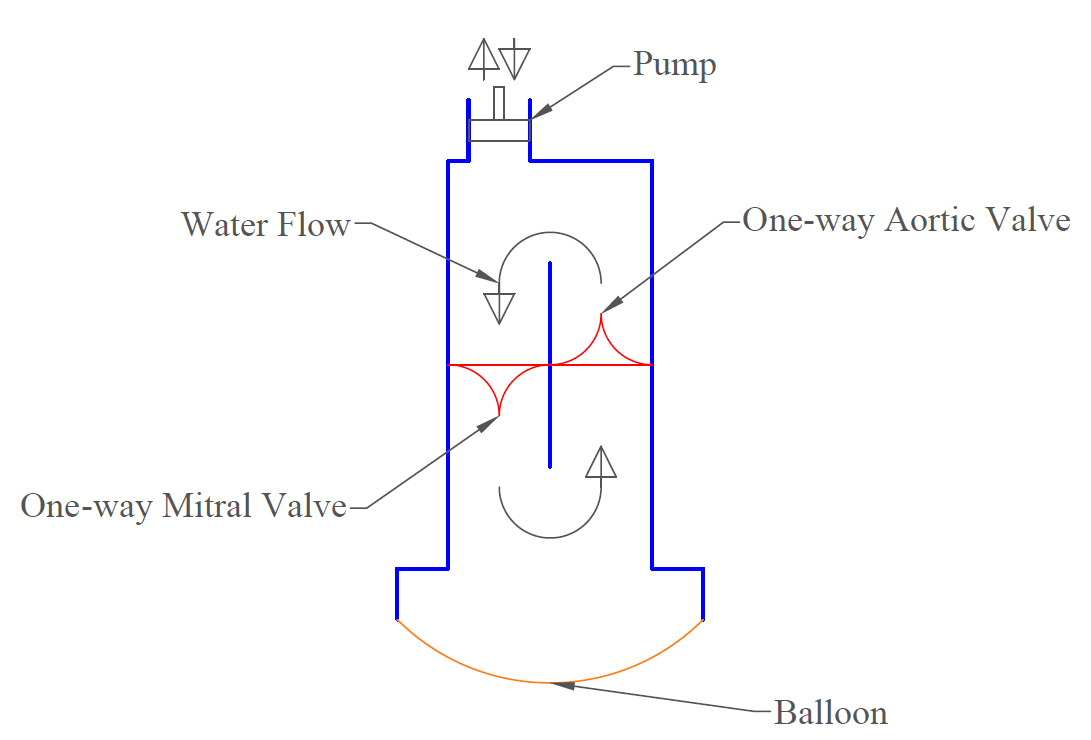
A modular, pulsatile heart simulator is currently being developed by the HVPL to create an adaptable testing platform for patient-specific prostheses. This device is anticipated to revolutionize heart valve testing and provide an unmatched preliminary analysis of the percutaneous valve implantation procedure.
Unfortunately, this device is still in the early stages of its development and due to its nature as a protected intellectual product, further details or imagery cannot be provided publicly at this time.